The Haber process, named after the German chemist Fritz Haber, is a chemical process used to synthesize ammonia (NH3) from nitrogen (N2) and hydrogen (H2) gases. It is one of the most important industrial processes for the production of ammonia, which is a key component in the production of fertilizers, explosives, and various other chemical compounds.
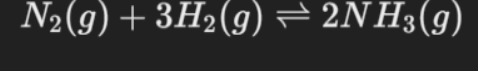
In this process, nitrogen gas (N2) and hydrogen gas (H2) are reacted in the presence of a catalyst at high temperature and pressure to produce ammonia gas (NH3). The reaction is exothermic, meaning it releases heat.
Key features of the Haber process include:
Catalyst: The reaction is catalyzed by a catalyst, typically iron with small amounts of other promoters such as potassium oxide or aluminum oxide. The catalyst facilitates the reaction and increases the rate of ammonia production.
High Pressure: The reaction is conducted at high pressure, typically ranging from 100 to 250 atmospheres (atm). High pressure helps to shift the equilibrium of the reaction towards the formation of ammonia, increasing the yield of ammonia.
High Temperature: The reaction is also conducted at high temperature, typically around 400 to 500 degrees Celsius (°C). While high temperature helps to increase the rate of reaction, it can also decrease the equilibrium yield of ammonia. Therefore, a compromise temperature is chosen to balance the rate of reaction and the equilibrium yield.
Recycling Unreacted Gases: To improve the efficiency of the process and maximize the yield of ammonia, unreacted nitrogen and hydrogen gases are recycled back into the reactor after separation from the ammonia product. This helps to ensure that as much of the reactants as possible are converted into product.
